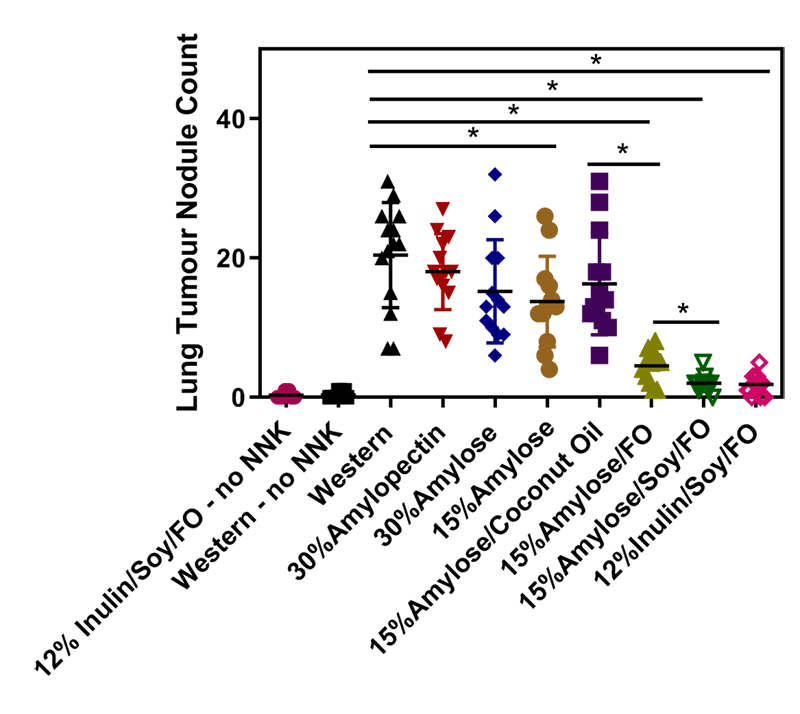Can Your Diet Affect Your Risk of Getting Cancer
Based on Otto Warburg’s finding in the 1920s that cancer cells take up and require far higher levels of glucose than normal cells, we asked, in our initial studies, if we could lower blood glucose (BG) levels sufficiently, through diet changes alone, to slow tumour growth. For these studies, we compared the effects of various isocaloric, low carbohydrate (L-CHO), high protein diets to Western diets on the growth of injected, rapidly growing murine or human cancer cell lines and on the incidence of spontaneous cancers in mice genetically predisposed to either breast or prostate cancer. We found that the L-CHO diets significantly lowered blood glucose levels and reduced the growth of subcutaneously injected murine squamous cell carcinoma and human colorectal carcinoma cell lines in mice1-3. Moreover, feeding L-CHO diets to transgenic mice (Her2/Neu) that spontaneously develop breast cancer dramatically reduced the appearance of tumours when compared to mice fed a Western diet. Additional long term studies demonstrated that L-CHO diets reduced the incidence of metastasis in a mouse prostate (TRAMP) cancer model and, when combined with the COX2 inhibitor, celecoxib, reduced 4T1 breast cancer metastasis2. Importantly, however, apart from a few notable exceptions (e.g., BRAC1 and 2, retinoblastoma), 90 to 95% of human cancers do not arise as a result of inherited genetic mutations (or by injecting tumour cells into us) but as a result of mutations that accrue with age due to carcinogen and/or chronic inflammation-induced DNA damage4. We therefore carried out more recent studies with a well established carcinogen-induced mouse cancer model in order to compare various dietary CHO, protein and fat components to see if we could further optimize a cancer prevention diet.
Specifically, we used a mouse model that closely mimics human cigarette smoke-induced lung cancer5, i.e., mice at 12 weeks of age (equivalent to “teenagers”) were put on different isocaloric diets for 2 weeks and then intraperitoneally injected with nicotine-derived nitrosamine ketone (NNK), a potent carcinogen involved in human cigarette-induced lung cancer5, to determine the effects of these diets on the number of lung nodules that developed over the next 5 months. As controls, we kept mice on both a Western and our best diet without injecting them with NNK. This was to determine the background level of lung nodules in this lung cancer prone mouse strain6. The lung nodule results are shown below in Fig1 and described in more detail in ref 7.
Lowering CHO levels, while increasing fat and protein to keep the chows isocaloric, significantly reduced lung nodules as well as postprandial (i.e., after eating) blood glucose levels in these mice. Diets containing soy protein (at 35% of total calories), amylose or inulin (at 15%) and fat (at 50%, 20% - 30% being fish oil (FO)) were the most effective at reducing lung nodules. These FO-containing diets (because of their omega 3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) increased plasma β-hydroxybutyric acid (a measure of ketosis), reduced plasma insulin and 8-isoprostane levels (a measure of oxidative stress), and suppressed lung PGE2 levels7. Taken together, our results suggest that L-CHO diets enriched in resistant starch (amylose) or soluble fibre (inulin) and containing high levels of soy protein and FO may reduce the incidence of lung cancer. Of note, resistant starch and soluble fibre are not converted into blood glucose in the small intestine like easily digestible starch, but rather transit to the large intestine where commensal bacteria (i.e, our microbiome) break them down to short chain fatty acids (SCFAs). These SCFAs help to maintain a healthy colon and also diffuse into the blood stream where they have an anti-inflammatory role, for example, in reducing the symptoms of asthma8. Also of note, soy protein in our studies appears to be superior to the animal protein, casein, in reducing NNK-induced lung nodules and our preliminary studies suggest this is not related to its isoflavone content.
One burning question from these studies, of course, is how extrapolatable these results are to humans. As a first foray into this, we compared plasma and red blood cell plasma membrane levels of arachidonic acid (AA, a pro-inflammatory, omega 6 fatty acid), EPA and DHA in our Western versus our 15%Amylose/Soy/FO fed mice with those in humans on a Western versus a low CHO diet supplemented with omega 3 fatty acids, i.e., 4.32 g of EPA (2.64 g) + DHA (1.68 g)/day. We found the levels of these fatty acids were not that dissimilar between the levels in our mice and in humans. Of course, simply comparing mouse and human levels of AA, EPA and DHA does not take into account possible differences in biological effects of these fatty acids in murine and human cells and comparative studies are ongoing in our lab.
Currently, to explore the universality of our findings, we are comparing a Western diet with our 15%Amylose/Soy/FO diet on preventing breast cancer, prostate cancer, colon cancer and myeloid dysplastic syndrome in mouse models. We are also in the early stages of a clinical trial looking to see if EPA + DHA supplements reduce pro-inflammatory markers in heavy smokers.
In summary, our work so far suggests that a simple diet change that lowers blood glucose and incorporates soy protein and FO may reduce the risk of developing cancer.
In summary, our work so far suggests that a simple diet change that lowers blood glucose and incorporates soy protein and FO may reduce the risk of developing cancer. Considering that “an ounce of prevention is worth a pound of cure”, it might be time that we place more emphasis on lifestyle changes to help prevent cancer.