
Implementing saline gargle sample collection for COVID-19 testing
One of the key pillars of pandemic response is testing to detect active cases. But, to achieve this tenet, BCCDC PHL had to build mass testing capacity to go from performing no COVID-19 tests in 2019 to thousands per day in 2020. Every public health lab in the world was also trying to do the same thing. By March, high testing demand created shortages throughout the worldwide supply chain for many products, including nasopharyngeal (NP) swabs—essential for testing.
At the same time, the Ministry of Forests, Lands, Natural Resources Operations and Rural Development approached the BCCDC to find a way to meet testing needs for tree planters. The tree planters would be heading into rural and remote regions of BC and the Ministry wanted to collect specimens from tree planters who showed COVID symptoms without having them entering the nearby remote communities, thereby protecting the communities.
However, the solution required a stable test medium that could withstand a long journey through possible extreme heat swings in the BC interior summer, and also a test collection method that didn’t rely upon using a NP swab or a healthcare provider to collect the sample. The solution also required components of the test collection system to be ubiquitous, and unlikely to be affected by supply chain issues. The race for an alternative was on.
We searched the literature and I thought back to my residency days working in the emergency department and other clinical environments for inspirations for a self-collection method – one that would be healthcare provider independent with a transport media that would suspend and preserve the virus, and allow for nucleic acid amplification. What came to mind were the little pink single-use saline vials that were present in pretty much all nursing stations. Saline was identified by the FDA as an acceptable transport media for the COVID-19 virus, SARS-CoV-2. In addition to saline, alcohol mouthwash and sterile water were other possibilities for oral rinses. Straight saliva had been evaluated by other groups. Obtaining an oral sample seemed to be a promising approach, but it also raised its own challenges of possible overgrowth from fungal and bacterial mouth flora.
We tested all three oral rinses: alcohol mouthwash, saline, and sterile water, all spiked with influenza viruses, as no one had been able to grow COVID-19 in culture yet. We found that saline performed very well in suspending influenza virus (which is also an enveloped virus, like coronavirus) while mouthwash inhibited the viral NAT assay. Water had no properties to prevent fungal and bacterial overgrowth.

Gargling with saline had the advantage of sampling the oropharynx, which was as close as we can get to the nasopharynx, the gold standard sample. In contrast to saliva, saline gargle samples are not mucoid and unlikely to require additional processing before its ready for NAT testing. So we lined up technologists who had agreed to have NP swabs collected along with saline gargle. We spiked these samples with influenza, and tested it against temperature and time. Again, we were able to demonstrate that saline gargle and NP swabs were equivalent in supporting the presence of spiked influenza viruses in the laboratory setting.
Around this time, our colleagues at BC’s Children’s and Women’s Hospitals Laboratory were also setting up a clinical comparison of sample types with COVID-19 positive patients to determine whether, NP, saliva, or throat swabs were preferable. We formed a collaboration and added saline gargle to the mix to evaluate it through a clinical trial. At this point, we were into the summer months and there were very few COVID-19 cases in BC. We reached out to our public health colleagues at Vancouver Coastal Health as well as at Fraser Health to identify potential COVID-19 positive patients who would be interested in participating in this study. Individuals who participated provided samples for the different sample types and all samples were tested for COVID-19 Dr. David Goldfarb’s determination slowly but steadily increased our sample size. He would follow up with willing positive patients, personally travelling across town to the patient’s home if that was what it took to progress the study. The outcome demonstrated that saline gargle was clinically equivalent to NP swabs (JCM publication: https://jcm.asm.org/content/early/2021/01/28/JCM.02427-20).
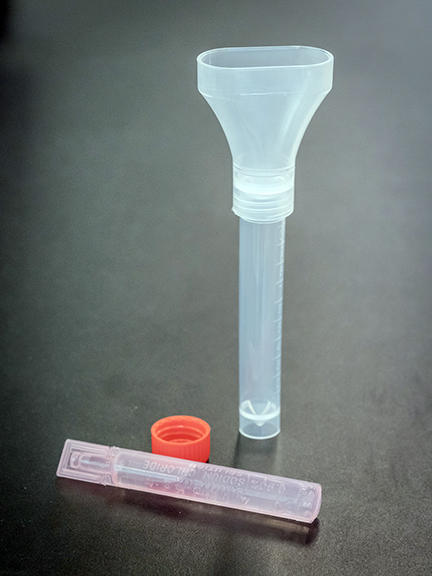
While the clinical trial was underway, the details around the appropriate collection system had not been solved. At this point, it was already September and the start of school was approaching. With rising case numbers, the thought of performing NP swabs on school aged children would be a barrier to the start of in-person classes. Public Health Services Authority (PHSA) Supply Chain used their extensive supplier contacts and expertise to identify a manufacturer in China that could produce a sterile tube with an attached funnel that could act as an appropriate collection system. These systems along with the saline gargle sample had to be evaluated. Again, we lined up volunteer technologists to test out these samples, along with testing and validating the collection systems at the BCCDC PHL.
The push to complete the clinical validation, and finding a suitable collection system, allowed the Provincial Laboratory Medicine Services to recommend the adoption of saline gargle for school-aged children and youth across BC. Saline gargle went live in BC on September 17, 2020 - one week after children returned to the classroom - allowing children to more easily provide samples for COVID-19 NAT testing at all COVID Collection Centres in BC.
This undertaking united a multi-disciplinary group of health care professionals from development to implementation. We had to ensure that all the COVID-19 testing laboratories in the province were able to validate saline gargle samples on the COVID-19 NAT assays and document that the work was completed. We had to quality-assure and standardize the collection method, testing and reporting across all health authorities in BC. In addition, operationalization of a new collection method had to be fast.
The communications team at BC Children’s Hospital developed some video instructions starring Dr. Goldfarb and his daughter.
A group was tasked with creating written collection instructions and accompanying collection video, updating guidance documents, training front-line staff, and sourcing supplies. Meghan McLennan from Provincial Laboratory Medicine Services, along with folks in PHSA Supply Chain, as well as all the public health-led Collection Centres across BC took on the operationalization of the saline gargle method. The communications team at BC Children’s Hospital developed some video instructions starring Dr. Goldfarb and his daughter. All this material became centralized on the BC Centre for Disease Control website and made publicly accessible (http://www.bccdc.ca/health-info/diseases-conditions/covid-19/testing/mouth-rinse-and-gargle).
By late November 2020, I had a phone call from the office of the Deputy Prime Minister, the Rt. Hon. Chrystia Freeland’s, requesting that we support the dissemination of the saline gargle sample type for COVID-19 NAT testing across the country.
Collection of saline gargle samples has since expanded to adults across BC testing sites. By late November 2020, I had a phone call from the office of the Deputy Prime Minister, the Rt. Hon. Chrystia Freeland’s, requesting that we support the dissemination of the saline gargle sample type for COVID-19 NAT testing across the country. The federal government sourced the collection tubes and saline vials from the suppliers we first identified for distribution across the provinces. Saline gargle has become the one of the standard sample types for testing COVID-19 in BC and in many centres across Canada and internationally. We are proud of how everyone pulled together to create this innovative and easy-to-use solution that will increase the uptake of testing and help detect COVID-19 cases in children and adults, and has become a standard of practice.

