
BC Children’s Hospital BioBank – Introducing Our New e-Consent Platform!
The BC Children’s Hospital BioBank (BCCHB) opened in January 2015 and is actively collecting biospecimens and clinical data from patients, who give their consent and who are seeking medical care at BC Children’s Hospital or BC Women’s Hospital. The BCCHB is in a unique situation of being able to collect diverse specimens from a wide variety of children and women for use in research.

Before patients participate and specimens are collected, patient or parental/guardian consent is obtained. The existing consent process informs the patient of the purpose, risks and benefits of the BioBank, among other information. In addition, children aged 7 years and older partake in an assent process where the child is informed about the research and their permission to participate is also requested. Informed consent for research is vital in achieving ethical standards, and for maintaining participant and public trust.
The original consent process for the BCCHB consisted of a traditional 11-page paper form with few visual aids, and feedback received through our patient and public engagement initiatives indicated that this consent form was not user friendly. Dr. Suzanne Vercauteren, Director of the BCCHB, and her team developed an electronic version of the consent in the hope to improve accessibility, comprehension, and engagement of pediatric participants. With the help of a grant received form the Clinical Research Support Unit (CRSU) at the BC Children’s Hospital Research Institute (BCCHR), this project has launched our new electronic consent (e-Consent) platform!
With the help of the BCCHR’s data management and IT team, we’ve adapted our traditional BCCHB consent forms into a modern, interactive, electronic format which is accessible on most electronic devices via an online link or QR code. Our multidisciplinary team of biobanking specialists, ethicists, patient experience scientists, a privacy/regulatory expert, a bioinformatician, an Emily Carr art student, and a UBC computer science co-op student provided input throughout the development process. In addition, patient/family representatives advised on all stages of the project, piloted the consent tool, and provided input from the end-user’s perspective. Focus groups were also conducted to assess overall participant satisfaction and user experience while navigating this new e-Consent. Patient and family representatives, including children and adolescents consistently indicated that the e-consent platform provided clear information.
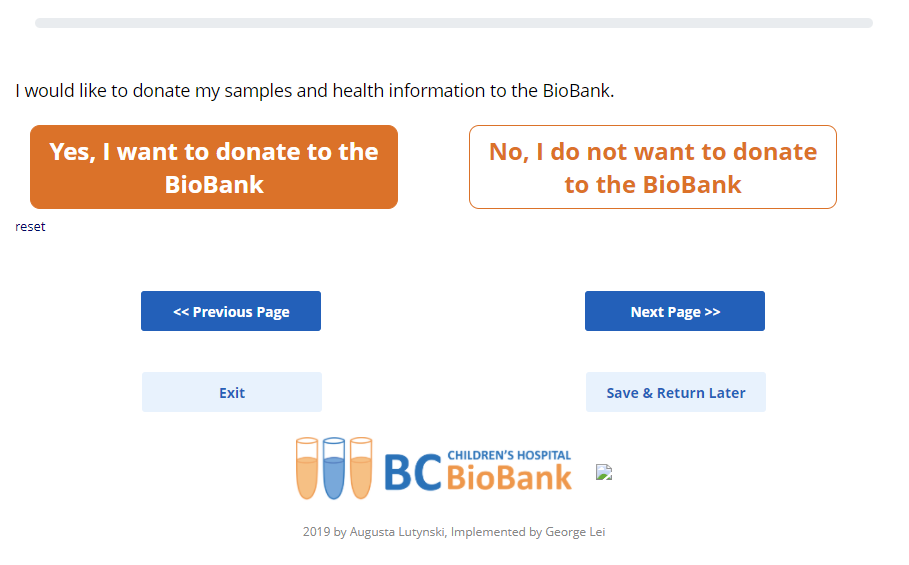
We are excited to announce that in November 2020, we received REB approval, and the e-Consent has now been implemented for use! This e-Consent increases accessibility and facilitates the resumption of research recruitment on the C&W campus following the COVID-19 pandemic shutdown without the need for face-to-face interactions. In addition, we hope that this platform provides better patient information as it is more user friendly and can tailor to individual needs for information compared to the original paper consent form.

