COVID-19-associated Heart Injury: How does it Happen?
Drs. Bruce McManus and Paul Hanson at the UBC Centre for Heart Lung Innovation in affiliation with UBC Pathology and Laboratory Medicine have studied virus-associated heart failure for several decades. Although COVID-19 is primarily recognized as a respiratory disease, ongoing global research implicates the heart as a central target of injury, which in turn causes significantly increased morbidity and mortality. Evidence of direct viral heart injury and indirect damage due to thromboembolic complications and cytokine storm have been observed. Myocarditis has also been reported. Consensus on mechanisms and timing remains to be achieved. Thus, our research aims to delineate the mechanism(s) of COVID-19-related cardiac injury.
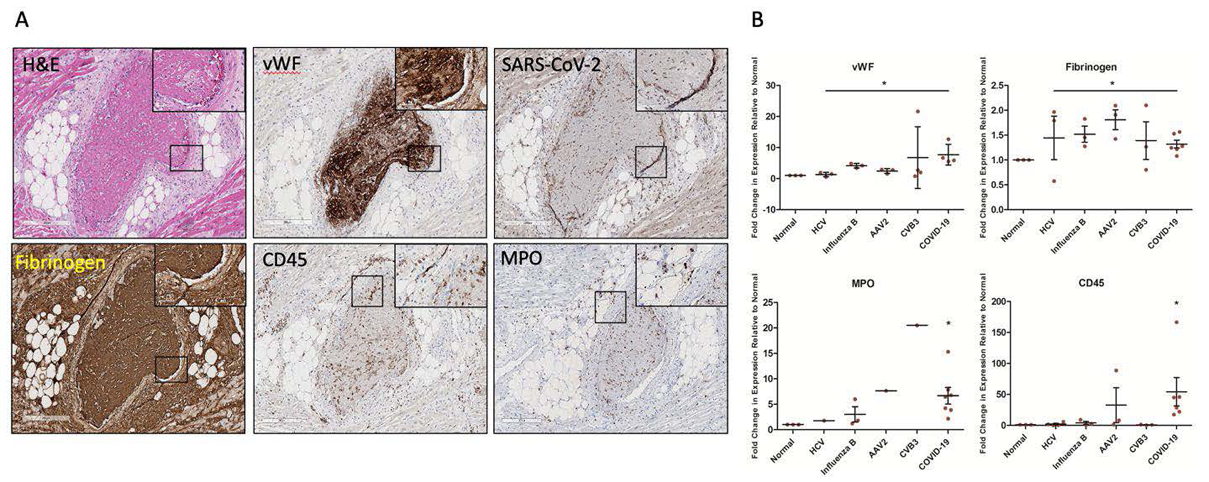
In concert with the University of Nebraska Medical Center, we have examined cardiac tissues from six COVID-19 positive decedents. We detected the SARS-CoV-2 spike protein in cardiomyocytes, within the interstitium, in the endothelial lining of arterioles and venules commonly associated with thrombosis, and within percolating adipocytes in the myocardium using RNAscope and immunohistochemical (IHC) staining. Further, we compared the COVID IHC results for markers of direct viral injury, thrombosis and inflammation to other common etiologies of virus-related heart failure from heart transplant and autopsy patients at St. Paul’s Hospital and to healthy controls via RNAscope. Relative to the other viruses, COVID-19 had increased markers of endothelial damage (von Willebrand factor, vWF), thrombosis (visually on H&E, and via CD61 and fibrinogen staining) and presence of Neutrophil Extracellular Traps (NETs) via myeloperoxidase (MPO). NETs were detected throughout the myocardium of all COVID-19 patients, within thrombi, and in large aggregated masses within the left and right ventricles, an apparent singularity of COVID-19. In line with IHC data, GeoMX® digital spatial profiling of over 1800 genes determined that thrombosis-related gene expression was significantly increased in COVID-19 patients relative to the other viral-infected tissues and healthy controls.
Our initial findings indicate endothelial damage, resultant thrombosis and NETs as defining features of COVID-19-associated cardiac injury. We are currently analyzing heart tissues from an additional 17 COVID-19 decedents to confirm and extend our findings in a larger cohort. Collectively, our study promises to identify key mechanism(s) underlying COVID-19-associated cardiac injury and may eventually contribute to diagnostic and therapeutic target development for COVID-19 patients with cardiac injury.

