You are chopping up vegetables and you cut yourself with a kitchen knife. What you don’t realize, as you reach for your anti-bacterial cream and band-Aid, is that you have just started a breathtaking battle that you hardly notice! First off, the damaged skin (epidermal) cells and underlying immune cells (specifically white blood cells called mast cells and macrophages in the dermis), release a cocktail of molecules (see Fig 1). Importantly, different cocktails are released in response to different microbial invaders. So, for example, different molecules are released if the entering microbe is a virus, a bacterium or yeast. Within seconds your capillaries dilate in response to some of the molecules and your plasma starts leaking out of your blood stream to wash away the microbes. This makes your cut feel hot and look red and swollen.
The first cells to be recruited (within 60 min) into your cut from your blood stream are white blood cells called neutrophils. These cells explode on entry, releasing intracellular molecules that promote inflammation, like reactive oxygen species (ROS) that damage the invaders. As the neutrophils die, they form the pus we sometimes see. The next immune cells to arrive into the cut are white blood cells called monocytes (within 24 hrs of getting cut) and, upon leaving the blood stream, they turn into macrophages. If the neutrophils have already won the battle, the remaining neutrophils don’t explode but die quietly. When they die quietly they trigger the incoming monocytes to become healer macrophages and they quietly gobble up the dead microbes and dead neutrophils and secrete anti-inflammatory molecules to begin the healing process. At the same time, some of these macrophages go into lymph vessels with pieces of the invaders to tell helper T cells in the nearest lymph node that we got it handled and you don’t have to expend a lot of energy expanding specific T cells or B cells that recognise the invaders to help out.
If, on the other hand, the neutrophils are losing the battle (bacteria can divide every 20 minutes!) and are still dying by exploding, the incoming monocytes become killer macrophages and release more ROS and pro-inflammatory molecules and take pieces of the invaders to the nearest lymph node to trigger the expansion of T or B cells that recognise the piece of invader (takes about 4 days for these adaptive immune cells to expand up enough to help).
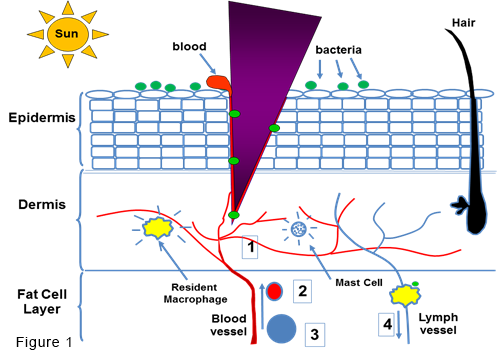
Fig 1. When you cut yourself with the purple knife blade, the entry of a specific microbe triggers the epidermal cells, mast cells and macrophages to release a cocktail of molecules that (1) make the blood vessels expand (dilate) and leak plasma into the wound, causing it to swell, (2) attract in white blood cells called neutrophils (shown as a red cell) and, later, monocytes (shown as a blue cell), which become macrophages (yellow cell) in the wound. Later, macrophages with a piece of the microbe go to the nearest lymph node (4) to tell T cells to expand up if you are losing the battle or not if you have killed off the microbes.
What we have just described is an acute inflammatory response, which makes our immune system (composed mainly of white blood cells) our best friend. Our immune system’s job is to fight off infections, help repair damaged tissues and quietly gobble up the 50 billion cells that normally die in our body every day. However, it can also be our worst enemy, causing allergies, autoimmune diseases and cancer.
Chronic Inflammation
When these damaged cells enter the healing phase, they are nurtured to grow and some of these ROS-induced DNA-damaged cells can progress, with subsequent waves of inflammation and healing, to become full blown cancer cells.
What if the acute inflammation described above is not resolved quickly, either because the microbe is difficult to destroy or the instigator of the inflammation is not a microbe at all but something that cannot be eliminated, like asbestos or constant cigarette smoke in our lungs? This leads to chronic inflammation (CI), where neutrophil and macrophage generated ROS damages our own cells. That is why it is so important to switch to healing as soon as microbes are destroyed. This continuous damage often leads to the generation of antibodies and/or killer T cells that can cause autoimmune diseases, like multiple sclerosis or lupus. As well, CI often consists of cycles of inflammation and healing. During the inflammatory part of the cycle, immune cell-generated ROS causes mutations in the DNA of our own cells. When these damaged cells enter the healing phase, they are nurtured to grow and some of these ROS-induced DNA-damaged cells can progress, with subsequent waves of inflammation and healing, to become full blown cancer cells.
So if acute inflammation is not turned off in a timely fashion it can lead to CI that in turn can result in autoimmune diseases and cancer. But the role of our immune system in promoting cancer doesn’t stop there. While our immune system is usually very effective at eliminating most cancers before they get a foot hold, some tumours secrete molecules to “convince” incoming immune cells they are just a wound that needs healing and our immune cells become co-opted to actually help them grow and survive. Understanding all the factors that tumour cells express to suppress their own destruction by immune cells has led to some recent breakthroughs in immunotherapy. For example, antibodies like Nivolumab and Ipilimumab bind and block the action of molecules that tumours express to prevent T cells from killing them.
Inflammaging
Interestingly, as we get older our levels of CI increase, in part because we accumulate cells that no longer carry out their assigned functions (senescent cells) and these cells produce high levels of ROS and other pro-inflammatory molecules. In fact, the association between aging and increased CI is so well established that it is called “inflammaging” and just about all the infirmities associated with aging can be attributed to this increase in CI, including type 2 diabetes, osteoporosis, arthritis, multiple sclerosis, Alzheimer’s disease, Parkinson’s, cardiovascular disease and, of course, cancer. As well, as we age our immune system becomes less able to respond to microbial challenges (and immunizations). However, we and others have found that there is a great deal of person-to-person variation, not only in the levels of CI as we age but in our ability to respond to bacterial or viral challenges. So what is responsible for this variation? Genetics certainly play a role but simply screening our genes will not reveal a person’s level of CI because many other variables are involved, such as injuries, alcohol and tobacco use, exercise, diet, stress levels and even the composition of the microbes that live in our intestines (our microbiome).
Development of “Inflammaging” and Immune Function Assays
If you or anyone you know would like to help us complete this important inflammaging study we would greatly appreciate it. Please contact us at gkrystal@bccrc.ca if you can spare the small amount of time involved.
We hypothesized that those with high levels of CI and a poor anti-viral response might be at higher risk of developing cancer and so developed sensitive tests to identify these people using very small samples of blood. To date we have tested over 200 healthy volunteers in 3 different age categories with our assays and are now comparing results obtained with them to those from heavy people and heavy smokers. Our results show, as expected, that some markers of CI increase and anti-viral responses decrease as we age but, importantly, there is significant person-to-person variation, with some older people showing lower levels of CI and better anti-viral responses than some much younger people. Importantly, we have found that high levels of CI do not predict a high or low response to a viral challenge, indicating that measuring both are important to gain a full picture of a person’s immune status. We feel these studies should help to identify individuals at high risk of developing cancer. We are still actively recruiting volunteers with a body mass index (BMI) over 40. If you or anyone you know would like to help us complete this important inflammaging study we would greatly appreciate it. Please contact us at gkrystal@bccrc.ca if you can spare the small amount of time involved.
Our lab is also very interested in the effect of diet and exercise on CI and cancer and we have found that mice that exercise and have low carbohydrate diets supplemented with omega 3 fatty acids have a markedly reduced incidence of tobacco-induced lung cancer (compared to mice on a Western diet). We are currently exploring the mechanisms underlying the dramatic effect that these diets have on cancer prevention and looking to see if this applies to other cancers as well.
Our results are covered in more detail in: Effect of age on chronic inflammation and responsiveness to bacterial and viral challenges. Elisia I, Lam V, Hofs E, Li MY, Hay M, Cho B, Brooks-Wilson A, Rosin M, Bu L, Jia W, Krystal G. PLoS One. 2017 Nov 29;12(11):e0188881.